Abstract
Objectives The safety and efficacy of sirukumab, an anti-interleukin-6 (IL-6) monoclonal antibody, were evaluated in a 2-part, placebo-controlled phase II study of patients with active rheumatoid arthritis (RA) despite methotrexate therapy.
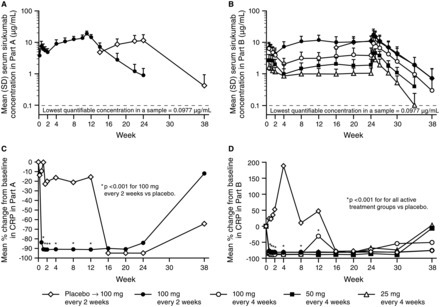
Methods In Part A (proof-of-concept), 36 patients were randomised to placebo or sirukumab 100 mg every 2 weeks (q2w) through week 10, with crossover treatment during weeks 12–22. In Part B (dose finding), 151 patients were randomised to sirukumab (100 mg q2w, 100 mg q4w, 50 mg q4w, or 25 mg q4w) through week 24, or placebo through week 10 with crossover to sirukumab 100 mg q2w (weeks 12–24). The proportion of patients with an American College of Rheumatology 50 (ACR50) response and the change from baseline in the 28-joint count disease activity score using C-reactive protein (DAS28-CRP) were determined. Safety was evaluated through week 38 in both parts.
Results The primary endpoint (ACR50 at week 12 in Part B) was achieved only with sirukumab 100 mg q2w versus placebo (26.7% vs 3.3%; p=0.026). Greater improvements in mean DAS28-CRP at week 12 were observed with sirukumab 100 mg q2w versus placebo in Parts A (2.1 vs 0.6, p<0.001) and B (2.2 vs 1.1; p<0.001). The incidence of adverse events (AEs) was similar for sirukumab-treated and placebo-treated patients through week 12 in Part A (70.6% and 63.2%, respectively) and B (67.8% and 66.7%, respectively). Infections were the most common type of AE; one death occurred (Part B, sirukumab 100 mg q2w, brain aneurysm).
Conclusions Sirukumab-treated patients experienced improvements in the signs/symptoms of RA. Safety results through 38 weeks were consistent with other IL-6 inhibitors.
Trial registration number NCT00718718.
Via
Krishan Maggon



 Your new post is loading...
Your new post is loading...